Compound Z Contains Carbon Hydrogen And Oxygen . For example, water, with two hydrogen atoms and one oxygen atom per. What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. How do you find molecular formula of a compound? If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen by subtracting the mass of carbon and hydrogen from the total mass of the original. When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. The subscript is written only if the number of atoms is greater than 1. How do empirical formulas and molecular formulas differ? Chemical formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced. In chemistry, combustion analysis is a quantitative analysis used to determine the empirical.
from www.chegg.com
The subscript is written only if the number of atoms is greater than 1. How do empirical formulas and molecular formulas differ? If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen by subtracting the mass of carbon and hydrogen from the total mass of the original. How do you find molecular formula of a compound? For example, water, with two hydrogen atoms and one oxygen atom per. In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. Chemical formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced. When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon.
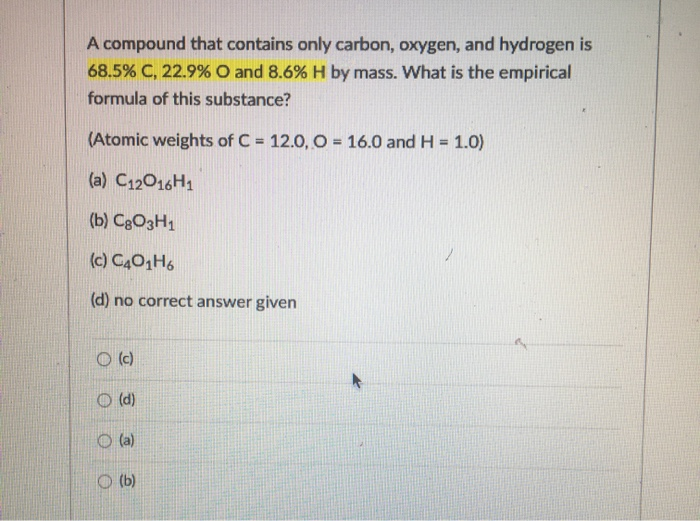
Solved A compound that contains only carbon, oxygen, and
Compound Z Contains Carbon Hydrogen And Oxygen For example, water, with two hydrogen atoms and one oxygen atom per. How do empirical formulas and molecular formulas differ? When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen by subtracting the mass of carbon and hydrogen from the total mass of the original. The subscript is written only if the number of atoms is greater than 1. How do you find molecular formula of a compound? In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. Chemical formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced. For example, water, with two hydrogen atoms and one oxygen atom per.
From www.chegg.com
Solved A compound contains only carbon, hydrogen, and oxygen Compound Z Contains Carbon Hydrogen And Oxygen How do you find molecular formula of a compound? When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. For example, water, with two hydrogen atoms and one oxygen atom per. In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. How do empirical formulas and. Compound Z Contains Carbon Hydrogen And Oxygen.
From kunduz.com
[ANSWERED] 24 An organic compound contains carbon hydrogen and oxygen Compound Z Contains Carbon Hydrogen And Oxygen When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. How do empirical formulas and molecular formulas differ? What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. For example, water, with two hydrogen atoms and one oxygen. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.meritnation.com
an organic compound contains carbon hydrogen and oxygen 1 80 gram of Compound Z Contains Carbon Hydrogen And Oxygen The subscript is written only if the number of atoms is greater than 1. In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. If the compound contains oxygen as well as carbon and hydrogen, calculate. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.coursehero.com
[Solved] A compound containing only carbon, hydrogen, and oxygen is Compound Z Contains Carbon Hydrogen And Oxygen In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. How do empirical formulas and molecular formulas differ? When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.numerade.com
A compound contains, by mass, 48.64 carbon, 8.16 hydrogen, and 43.209 Compound Z Contains Carbon Hydrogen And Oxygen In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. How do you find molecular formula of a compound? Chemical formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced. For example, water, with two hydrogen atoms and one oxygen atom per. When a. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.numerade.com
SOLVED A compound contains only carbon, hydrogen, nitrogen, and oxygen Compound Z Contains Carbon Hydrogen And Oxygen If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen by subtracting the mass of carbon and hydrogen from the total mass of the original. How do empirical formulas and molecular formulas differ? How do you find molecular formula of a compound? When a compound containing carbon and hydrogen is subject to combustion. Compound Z Contains Carbon Hydrogen And Oxygen.
From stock.adobe.com
Elements and Compounds are compared in the molecular structure. Oxygen Compound Z Contains Carbon Hydrogen And Oxygen The subscript is written only if the number of atoms is greater than 1. When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. How do you find molecular formula of a compound? In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. How do empirical. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.sarthaks.com
The percentage composition of organic compound to contains 26.66carbon Compound Z Contains Carbon Hydrogen And Oxygen How do you find molecular formula of a compound? How do empirical formulas and molecular formulas differ? When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. The subscript is written only if the number of atoms is greater than 1. What is the molecular formula of a compound. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.youtube.com
An organic compound contains carbon, hydrogen and oxygen. Its elemental Compound Z Contains Carbon Hydrogen And Oxygen How do you find molecular formula of a compound? The subscript is written only if the number of atoms is greater than 1. How do empirical formulas and molecular formulas differ? In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. Chemical formulas tell you how many atoms of each element are in a compound, and empirical. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.chegg.com
Solved An unknown compound 2 (containing only carbon, Compound Z Contains Carbon Hydrogen And Oxygen The subscript is written only if the number of atoms is greater than 1. In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. How do you find molecular formula of a compound? For example, water,. Compound Z Contains Carbon Hydrogen And Oxygen.
From askfilo.com
An organic compound containing carbon, hydrogen and oxygen contains 52.20.. Compound Z Contains Carbon Hydrogen And Oxygen When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. How do empirical formulas and molecular formulas differ? The subscript is written only if the number of atoms is greater than 1. How do you find molecular formula of a compound? What is the molecular formula of a compound. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.numerade.com
SOLVED Determine the class of the compound, which contains carbon Compound Z Contains Carbon Hydrogen And Oxygen If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen by subtracting the mass of carbon and hydrogen from the total mass of the original. In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. How do empirical formulas and molecular formulas differ? Chemical formulas tell you how many atoms. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.numerade.com
SOLVED A compound containing carbon and hydrogen contains 40.25 of Compound Z Contains Carbon Hydrogen And Oxygen Chemical formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced. If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen by subtracting the mass of carbon and hydrogen from the total mass of the original. How do empirical formulas. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.coursehero.com
[Solved] 0.5 points A compound contains only carbon, hydrogen, and Compound Z Contains Carbon Hydrogen And Oxygen When a compound containing carbon and hydrogen is subject to combustion with oxygen in a special combustion apparatus all the carbon. What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. For example, water, with two hydrogen atoms and one oxygen atom per. How do you find molecular formula. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.numerade.com
SOLVED A compound that contains only carbon, hydrogen, and oxygen is Compound Z Contains Carbon Hydrogen And Oxygen Chemical formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced. The subscript is written only if the number of atoms is greater than 1. In chemistry, combustion analysis is a quantitative analysis used to determine the empirical. What is the molecular formula of a compound containing. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.chegg.com
Solved A compound that contains only carbon, oxygen, and Compound Z Contains Carbon Hydrogen And Oxygen If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen by subtracting the mass of carbon and hydrogen from the total mass of the original. What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. For example, water, with two hydrogen atoms. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.youtube.com
An organic compound contains 69 carbon and 4.8 hydrogen, the Compound Z Contains Carbon Hydrogen And Oxygen What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. The subscript is written only if the number of atoms is greater than 1. If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen by subtracting the mass of carbon and hydrogen. Compound Z Contains Carbon Hydrogen And Oxygen.
From www.chegg.com
Solved Problem 3 This compound contains carbon, hydrogen Compound Z Contains Carbon Hydrogen And Oxygen The subscript is written only if the number of atoms is greater than 1. How do empirical formulas and molecular formulas differ? What is the molecular formula of a compound containing only carbon and hydrogen if combustion of 1.05 g of the compound. If the compound contains oxygen as well as carbon and hydrogen, calculate the mass of the oxygen. Compound Z Contains Carbon Hydrogen And Oxygen.